JOHNSON & JOHNSON's SHOCKWAVE takeover
Apr 09, 2024JOHNSON & JOHNSON's SHOCKWAVE takeover
Recently, Johnson & Johnson Medical Technology once again demonstrated its determination to expand its cardiovascular product line, with the acquisition of Shockwave Medical at a high price of $13.1 billion, further consolidating its leading position in the cardiovascular field.
The deal follows the late 2022 acquisition of mini heart pump maker Abiomed,( a $16.6 billion acquisition), and the 23-year $400 million acquisition of Laminar, (a heart implant developer designed to reduce patients' long-term risk of stroke due to atrial fibrillation).
In less than two years, J&J's mergers and acquisitions in the space totaled more than $30 billion. After the successful acquisition, Johnson & Johnson is expected to displace Medtronic as the top global medical device company.

New breakthrough: New technology to help cardiovascular disease treatment
Johnson & Johnson Medical Technologies is treating Shockwave's pioneering (Intravascular Lithotripsy IVL) catheter portfolio as a ticket to its 13th priority platform, which will join its portfolio along with other products with annual sales exceeding $1 billion. The IVL is a minimally invasive device that uses sound energy to shatter hard, calcified blockages deep inside the coronary and surrounding arteries.

"We are excited about this transaction," Johnson & Johnson CEO Joaquin Duato said on a conference call with investors.
"The acquisition of Shockwave, along with Abiomed and Laminar, will complement Johnson & Johnson's global leadership in electrophysiology through Biosense Webster, together building a highly differentiated cardiovascular portfolio," Duato said. "When the Shockwave transaction closes, J&J Medical Technologies will be a category leader in four high-growth cardiovascular segments."
The companies said they plan to close the deal by the end of June at a price of $335 per share, funded by a combination of cash and debt. After that, Shockwave will operate as a subsidiary while keeping its business structure largely unchanged, a model similar to how J&J currently operates Abiomed.
Part of the reason is to prepare for the upcoming launch of Shockwave's R&D pipeline, which has grown fourfold in the past two years to 27 different projects, including 13 products.
The company already has U.S. and European approvals for coronary heart disease and peripheral artery disease, including markets above and below the knee - which make up 80 to 90 percent of the deal's total price tag - and J&J says more than 400,000 patients have been treated with endovascular lithotripsis since 2017. Going forward, Shockwave will focus on new indications such as carotid artery disease as well as structural heart disease.
In addition, Shockwave completed its acquisition of Neovasc and its Reducer system last year, which may be the first device designed to treat intractable chest pain. The hourglass-shaped implant slows the flow of blood from the heart's coronary sinus veins, giving oxygen more time to transfer to the heart muscle.
"We have no plans to integrate our sales organization," said Tim Schmid, global chairman of Johnson & Johnson Medical Technologies. "We believe we will have a greater voice in the cardiovascular community, but our plan is to let these organizations focus on growing their business... We have a much broader portfolio coming up in the IVL space, and we will need these dedicated resources to make sure we take advantage of this opportunity."
Shockwave's current CEO Doug Godshall will serve in an advisory role during the transition, while Chief Business Officer Isaac Zacharias will become the company's global president.
Zacharias will report to Michael Bodner, global head of cardiac Recovery at Johnson & Johnson, who also oversees Abiomed. The two companies are no strangers to cardiovascular clinicians: J&J estimates that Shockwave's IVL catheter and Abiomed's Impella pump are used in about 30% of extremely high-risk percutaneous coronary interventions.
Shockwave's annual sales in 2023 increased nearly 50% year-over-year to $730.2 million, covering its coronary heart disease and peripheral artery disease segments. In the two years before that, the company's annual revenue had more than doubled. In its most recent earnings report, the company forecast growth of more than 25 percent in 2024, with revenue eventually coming in between $9.1 billion and $9.3 billion.
But J&J thinks it can help. "When we integrated Shockwave, we saw numerous opportunities to accelerate the distribution of these innovative technologies and expand treatment options to meet the needs of more patients over time," Schmid said, citing Johnson & Johnson's international sales infrastructure and physician education capabilities. "All of this will open up opportunities for us to penetrate new markets and expand these critical treatment options."
Innovative Medical device leader: Shockwave Medical
According to statistics, in the top 100 global equipment companies list in 2023, Shockwave Medical research and development expenditure increased by 61.6% over 2021, accounting for about 16.7% of its income, and is one of the top 100 medical device companies with the highest research and development investment.
Based in California, the innovative medical device company has been developing and marketing advanced technologies for the treatment of calcification of the cardiovascular system since its founding in 2009. In April 2019, Shockwave Medical successfully listed on NASDAQ, marking its growing position in the global medical device industry.
The company is known for its breakthrough "Intravascular Shockwave" calcification treatment technology (Shockwave IVL), a minimally invasive catheter-based treatment specifically designed to treat calcified coronary artery disease (CAD) and peripheral artery disease (PAD). Shockwave IVL technology uses sound wave energy to smash calcified plaques inside blood vessels, providing a safer and more effective treatment option for patients.
Shockwave Medical's products, including the Shockwave C2 device, have been widely recognized in the international market and are known as the "terminator" of coronary artery calcification. In addition, Shockwave Medical has further expanded its cardiac device business and market for the treatment of intractable angina through the acquisition of Neovasc.
With China's aging population, rising incidence of chronic diseases such as obesity and diabetes, and increasing prevalence of coronary artery disease, the IVL market is expected to usher in rapid growth. According to Frost & Sullivan, the global IVL market for coronary artery disease has grown from $1.7 million in 2017 to $64.1 million in 2020 and is expected to reach $3.347 billion by 2030. In China, the potential of the IVL market is not to be underestimated, and is expected to grow from 225 million yuan in 2023 to 918 million yuan in 2025, with a compound growth rate of 37.9% from 2025 to 2030.
In 2021, Health Medical established a joint venture with Shockwave Medical to introduce Shockwave's intravascular shock wave calcification treatment technology, which has been approved by the National Drug Administration (NMPA) and listed in the domestic market, bringing the Gospel to more Chinese patients.
Johnson & Johnson's recent acquisitions inventory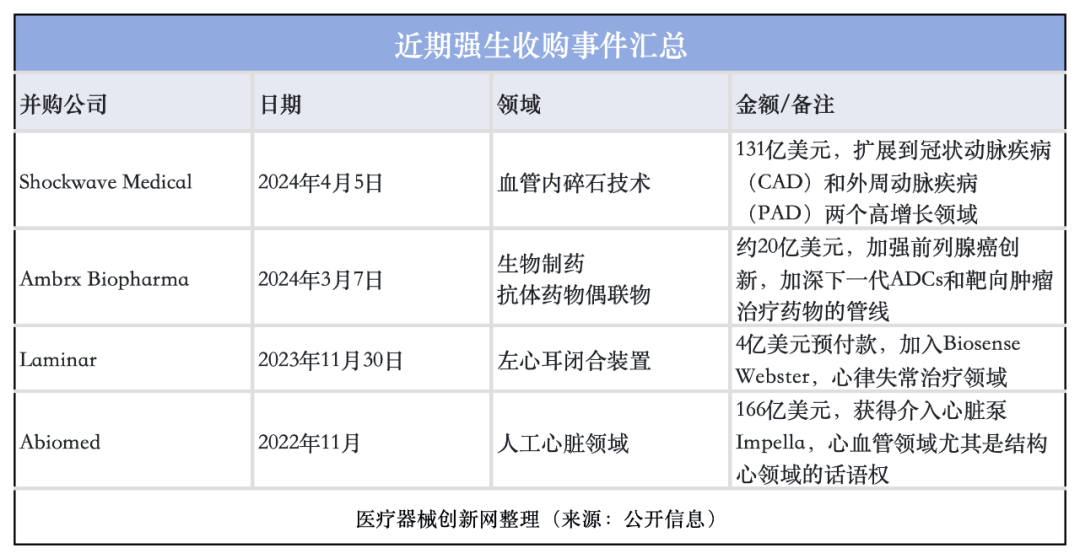
▲ Article source: Medical Device Innovation network
▲ Please mark the above source for reprinting


